Featured Article
CP-CTNet Cross Network Study: Lynch Syndrome
Ajay Bansal, MD, Eduardo Vilar-Sanchez, MD, PhD, Seema Khan, MD, and Kelly Benante, MPH

Inherited susceptibility to cancer involves defects in genes that normally function to suppress the formation of tumors. One such syndrome was recognized by Henry Lynch several decades ago based on clinical criteria, but is now known to be caused by inherited defects in genes that repair DNA damage, leading to the development of a variety of malignancies with onset early in life. People with Lynch Syndrome (LS) have a markedly increased incidence of cancers of the colorectum, uterus, and several other organs. These patients undergo invasive screening procedures such as colonoscopy, as well as risk-reducing surgery (colectomy, hysterectomy, oophorectomy) with their attendant morbidity and quality of life impact. When cancers do occur in carriers of LS, they are frequently biologically aggressive. Therefore, novel methods of cancer interception are needed.
We now understand that the immune system plays an important role in protecting us against cancer development, but cancer cells can develop mechanisms to evade immune surveillance. However, vaccines directed to antigens that are present on cancer cells can mobilize cellular immune responses to detect and destroy precancerous cells. Along these lines, many years of work from the laboratory of Dr. Jeffrey Schlom and colleagues at the NCI led to the development of vaccines that could target three well-recognized cellular proteins linked to carcinogenesis (carcinomebyonic antigen, CEA; brachyury; and the mucin MUC1). This vaccine combination has been tested in an advanced colon cancer population, where it was well tolerated. However, it has become clear through vaccine trials in multiple organ systems that the optimal use of vaccines may in fact be for cancer prevention in healthy high-risk individuals who have an intact immune system, rather than people with advanced cancer who are already immunocompromised. Therefore, a cancer prevention trial in the Lynch Syndrome population makes eminent sense.
Such a trial, with a primary endpoint of polyp prevention, will soon be opened through the five LAOs of CP-CTNet. Although Lynch Syndrome is the most common inherited gastrointestinal cancer syndrome, is still uncommon in the general population. Therefore, a major collaborative effort was needed that spanned centers specializing in care of patients with LS. The clinical design was discussed by a core group that included DCP, leaders of two LAOs (Northwestern and MD Anderson), and the two lead investigators in gastroenterology (Dr. Bansal, Study Chair) and medical oncology (Dr. Vilar-Sanchez, Study Co-Chair). Key biostatistics input was provided by Drs. KyungMann Kim and Jens Eickhoff from DMACC. Team members with different skill sets provided multi-dimensional input, with differences of opinion that needed to be resolved to reach a consensus. We benefited immensely from the experience of DCP (Drs. Szabo and Umar) and the LAO leaders (Drs. Khan and Brown), who had faced similar challenges in the design of other multicenter studies. These discussions helped to fine tune the scientific and logistical aspects. Given the location of team members across different time zones, the scheduling of video meetings required a significant amount of administrative support, and many conferences occurred late on Friday evenings, but the entire team was motivated by a common goal. Freewheeling discussions about the primary and secondary endpoints, the study population, the feasibility of biomarker assays, and other aspects of the study design allowed us to design a well-powered study that all judged to be feasible, and all agreed would lead to invaluable new information about immunoprevention not only for genetically susceptible individuals, but also those at high risk for sporadic cancer. To determine the size of the study, the two study chairs reached out to world-renowned experts in Lynch Syndrome. We also included an immunologist (Dr. Oliveira Finn), experienced in vaccine-based trials, to help us design the immunological endpoints.
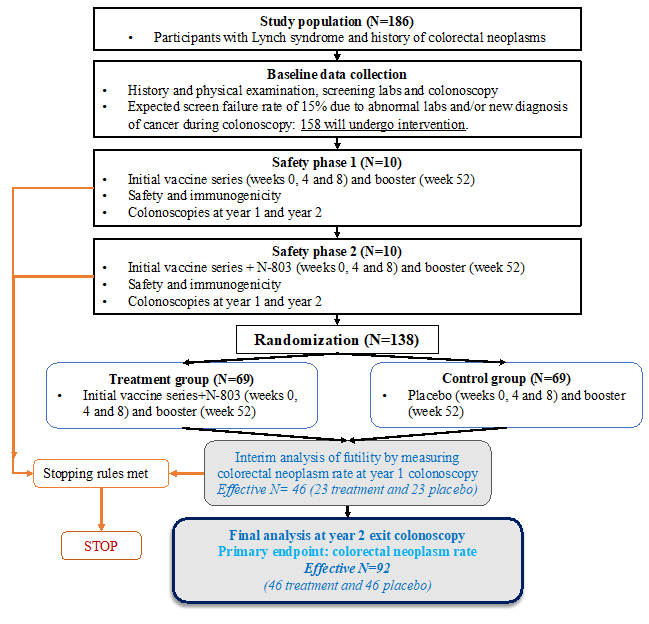
Once we ironed out the basic design, we organized two town hall style meetings to present our study concept and design to all of the investigators within the five LAOs. This gave us an opportunity to learn from the experience of the investigators who care for patients with Lynch Syndrome on a daily basis and have participated in previous cancer prevention trials. The result was a trial concept that was approved by DCP for protocol development. We then re-engaged with all of the sites within the five LAOs to write a study protocol which would enable us to conduct this national study across 14 different sites. These iterative interactions led us to broaden the scope of our study to include questionnaires to understand the participants' behavior as they enroll in this vaccine trial and adhere to its prescribed interventions. Several other discussions led us to include important biomarker endpoints, such as sequencing of immune-related genes and the potential effects of the vaccine on cancer stem cells.
A significant challenge has been the design of a process to manage biologic samples collected during the trial: in particular, the need to isolate peripheral blood mononuclear cells to measure the immune effects of the vaccine. These cells are easily destroyed during the isolation process, which requires that fresh blood must be shipped in real time from all the participating sites to the central processing site. The shipping costs also add budgetary complexities. We came up with the solution of storing pre-labeled kits on site to streamline specimen shipping. Also, for specimens that do not need to be shipped real-time, we decided to store them at individual sites, under similar conditions, to be batch-shipped at a later date to specific laboratories for biomarker analysis.
Putting this trial together has been a tremendous exercise in communication and collaboration. We are very grateful for the enthusiasm of all participating LAOs, investigators, and study teams, as well as the support and efforts of the NCI Division of Cancer Prevention, DMACC, ImmunityBio, CCSA, and MRIGlobal. Now that we are close to opening the trial, our next great challenge is to ensure successful enrollment and compliance with the protocol. We also hope that the lessons we have learned throughout the development of this study will inform future cross-network trials and SOPs.
DMACC Updates
Data Management and Reporting Unit
System Variable Attribute Report (SVAR) Process and Study Builds in Medidata Rave for New Studies
During this quarter, DMACC started initial drafts of three SVARs (INT22-09-01, UMI22-09-01, and UMI22-09-02) and plans to finalize these drafts to send to the LAOs for review in the near future. DMACC also continued to make progress on three SVARs (INT21-05-01, UMI21-05-01, and UWI20-04-01) and finalized three SVARs (MDA20-02-01, UAZ21-07-01, and UWI21-06-01). During the next quarter, DMACC plans to start an initial draft of one additional SVAR (UAZ22-10-01). Since mid-June, five study builds have been pushed to production in Rave (UWI20-00-01, MDA21-06-01, UAZ21-06-01, MDA20-01-01, and UWI21-06-01). Work is ongoing on four study builds, with two on track to be completed by the end of September (MDA20-02-01 and UAZ21-07-01).
A data visualization of DMACC’s real-time, detailed SVAR and study build status report has been developed, and work is underway to share this visualization with the LAOs via a dashboard item page on the Portal Gateway.
Virtual Specimen Repository
The CP-CTNet Virtual Specimen Repository framework was developed and tested this quarter, with initial deployment tentatively scheduled for the end of October. Data for studies UAZ20-01-01 and NWU20-02-01 continued to funnel to DMACC in real time. DMACC developed a prototype for a sample study-specific specimen inventory expectancy vs. actual specimen collection report as per LAO request, which was presented to the Virtual Specimen Repository Group at the September 26, 2022 meeting.
Meetings
Study Initiation Meetings (SIMs) were held for five studies during this quarter, including MDA20-02-01, UWI21-06-01, UAZ20-01-01, UAZ21-07-01, and INT21-05-01. A Cross-Network Collaboration (CNC) call was held on September 1, 2022.
Documentation
DCP and DMACC collaborated to update the following documentation on CP-CTNet-DMACC.org:
- SOP 01-02 Study Initiation Meeting
- CP-CTNet Study Initiation Meeting Report Template
- SOP 02-02 Reporting Protocol Deviations
- QKREFGD05 Protocol Deviation Reporting for LAOs and AOs
- QKREFGD06 Protocol Deviation Review for LAOs
- QKREFGD07 Protocol Deviation Review for DCP
- SOP 02-03 Electronic Case Report Form Development
- SVAR Template
- USRMAN01 CP-CTNet Stars User Guide
- QKREFGD02 Summary of Enrollment Process
- QKREFGD08 CP-CTNet Treatment ID Information
- REFGD03 CP-CTNet Master Data Management Plan
- QKREFGD04 DMACC Systems and Resources Quick Reference Guide
Educational Content
DMACC offered 13 training sessions for CP-CTNet members during this quarter. Training session topics included the Medidata Rave recruitment journal, the CP-CTNet DMACC website and Portal Gateway, the protocol deviation reporting and review process in Medidata Rave, Medidata Rave reports, and the Stars and Medidata Rave enrollment flow. To see a list of upcoming live trainings and to register, go to the Training Registration page on the Portal Gateway.
DMACC updated six video tutorials and associated video transcripts to guide the use of the updated Stars Registration/Randomization system during the enrollment process. These video resources are available on the Stars and Virtual Training pages on the Portal Gateway. Three new video tutorials and associated transcripts were also created to guide the process of reporting and reviewing protocol deviations in Medidata Rave for LAOs, AOs, and DCP. These video resources will be available soon on the Medidata Rave and Virtual Training pages on the Portal Gateway.
During the next quarter, we plan to provide training resources to CP-CTNet members to guide the use of the newly released Audit System during DMACC quality assurance audits.
Website and Portal Gateway Update
DMACC is always working to keep CP-CTNet-DMACC.org updated with the latest information for CP-CTNet sites, network colleagues, collaborators, and the public. Since July, several new funding opportunities, news posts, and document resources have been added. Many links and resources have been updated on the password-protected CP-CTNet DMACC Portal Gateway as well, including the availability of additional translated Central Institutional Review Board (CIRB) Short Form Consents and Certificates of Translation on the Document Library page.
DMACC continued to explore more data visualization options and reports that will be added to the Portal Gateway to monitor study progress and key metrics.
The Website Review Committee met in June and August.
Clinical Trials Auditing Unit
July was a busy and exciting month for the Audit Team as they were able to perform their first in-person, on-site audits at LAO Northwestern University (July 13-15) and their AO, Cedars-Sinai Medical Center (July 27-28). Three CP-CTNet protocols (NWU20-01-03, NWU20-02-01, and NWU20-02-02) were the focus of these audits. Northwestern is an accruing LAO for NWU20-02-01, a surgical window of opportunity study comparing megestrol acetate to megestrol acetate combined with metformin for endometrial intraepithelial neoplasia, and NWU20-02-02, a study that tests the use of atorvastatin to lower the risk of colon cancer in individuals with longstanding ulcerative colitis. Northwestern is a non-accruing LAO for NWU20-01-03, which is investigating the role of lisinopril in preventing the progression of non-alcoholic fatty liver disease. Dr. Ju Dong Yang at Cedars-Sinai recently took over as protocol PI for this study. It was a great pleasure to meet LAO PI, Dr. Seema Khan, and her team at Northwestern and Protocol PI, Dr. Yang, and his team at Cedars-Sinai.


The Audit System application has been revised to implement several user interface and workflow improvements requested by DCP and the LAOs. These enhancements improve the functionality and user experience for each role that accesses the system during DMACC quality assurance audits.
The Audit Team is looking forward to hosting a webinar on October 18, 2022 that focuses on tips for quality assurance audits and LAO oversight. More information regarding this webinar will be distributed throughout the network soon.
Administrative and Coordinating Unit
Our team continues to disseminate important information to the greater CP-CTNet audience via email and updates to the CP-CTNet DMACC website, and to provide administrative and coordinating support to CP-CTNet. Brainstorming new and exciting content for the quarterly CP-CTNet newsletter also keeps us busy! In addition, our Statistics Unit is working on the cross-network studies: INT21-05-01, A Phase IIb Clinical Trial of the Multitargeted Recombinant Adenovirus 5 (CEA/MUC1/Brachyury) Vaccines (Tri-Ad5) and Il-15 Superagonist N-803 in Lynch Syndrome, and INT22-09-01, Randomized Trial of Apalutamide in Non-Muscle Invasive Bladder Cancer.
Our team was also instrumental in organizing the upcoming Data and Safety Monitoring Board (DSMB) meeting. The DSMB will serve a very important role in assuring participant safety and the quality of our cross-network trials. The DSMB will regularly monitor the data from the studies, review and assess the performance of its operations, and make recommendations, as appropriate, to NCI/DCP. Planning for the next I-SCORE meeting will begin in November. If anyone has any thoughts, ideas, suggested guest speakers, or topics you’d like to discuss for I-SCORE 2023, please email Admin_CP-CTNet@frontierscience.org.
Equity, Diversity, and Inclusion (EDI) Committee Updates
EDI for Education
The committee, led by Dr. Adam Murphy, completed a learning needs assessment by the LAOs and AOs. Dr. Murphy presented a webinar entitled Diversity in Clinical Trials on August 2, 2022.
EDI in Clinical Trials
The committee, led by Dr. Julie Bauman, completed edits to the DCP CP-CTNet Chemoprevention Protocol Template addressing the exclusion and inclusion criteria. This template was reviewed by DCP, uploaded to the DCP CP-CTNet website for use, and sent to the LAOs.
EDI Staffing
The committee, led by Dr. Howard Bailey, has developed a survey to evaluate the climate of CP-CTNet. Responses to the survey were due August 31, 2022. The committee is looking forward to reviewing the results.
Staff Highlight
About the Protocol Information Office (PIO) of DCP
The Protocol and Information Office (PIO) was created to serve as the central hub of information processing for maintaining the official files of all documents in which DCP is involved.
PIO provides effectual assistance, which minimizes the burden related to clinical trial development and management on DCP staff and site staff that process clinical trials; monitors study developments from the establishment of ideas through quality review approval, implementation, and completion; and manages and maintains the completeness, accuracy, and integrity of clinical trials information in DCP databases and PIO portal.
In addition to CP-CTNet, PIO also supports:
- 2012 Consortia Program
- US-Latin American-Caribbean Clinical Trial Network (ULAC-NET)
- National Cancer Institute Community Oncology Research Program (NCORP)
PIO manages the entire protocol process, following each protocol through the steps of submission, review, and approval. Amendment changes, correspondences, and receipt of a final published report are additional tasks of the PIO throughout the lifecycle of the protocol.
Who are the main staff members and their individual responsibilities within PIO that work on CP-CTNet projects? List one fun fact that members of CP-CTNet may not know about you.
- Troy Budd, PMP, C.C.R.P.: The head of PIO
- Evelyn Taylor: Loves everything football and vintage cars made between 1965 and 1968.
- Ann Small: An early retiree. Previously retiring as a Tax Clearance Supervisor from the Board of Inland Revenue, Ministry of Finance, Republic of Trinidad and Tobago.
- Evelyn and Ann abstract key information and milestones in PIO-Clinical Trials System throughout the protocol lifecycle from concept receipt to protocol terminal status.
For more information about PIO, as well as instructions, templates, and forms, visit the DCP PIO website.
Reminder: When contacting PIO, also use NCI_DCP_PIO@mail.nih.gov to help ensure a quick and efficient reply.
Research Funding Opportunities
Grants and Awards
For a list of funding opportunities, check the Funding Opportunities page on the CP-CTNet DMACC website.
The Cancer Prevention and Control Clinical Trials Planning Grant Program (R34/U34) held a Pre-Application Webinar on August 8, 2022, from 2:00pm – 3:30pm Eastern Time. More information can be found here: PAR-22-173 "Cancer Prevention and Control Clinical Trials Planning Grant Program (R34 Clinical Trials Optional)" and PAR-22-174 "Cancer Prevention and Control Clinical Trials Planning Grant Program (U34 Clinical Trials Optional)". Feel free to reach out with questions to DCPDCCPSCT@mail.nih.gov.
Please be aware of a new funding opportunity from the NCI Small Business Innovation Research (SBIR) program, NIH/NCI 453 – Digital Tools to Integrate Cancer Prevention Within Primary Care. This opportunity was developed in response to the Cancer Prevention in Primary Care Roundtable, convened by DCP to determine barriers for integrating cancer prevention within primary care and discuss potential opportunities to overcome these barriers. This solicitation is supported by the NCI SBIR program, an office within NCI that supports the development and commercialization of novel technologies. Please send the solicitation to any networks you think would be interested in applying. The solicitation will be open to applications through November 4, 2022.
The Cancer Moonshot Scholars Program seeks to diversify the NCI R01 portfolio by enhancing the number of applications submitted by Early Stage Investigators from diverse backgrounds, including those from groups identified as underrepresented in the biomedical, clinical, behavioral, and social sciences research workforce. In addition, the program seeks to increase the diversity of thought and approach to cancer research. Individuals from underrepresented groups are especially encouraged to work with their respective institutions to apply. The first due date is November 8, 2022. NCI expects to support 45 new R01s through this program over the next three years.
Prospective applicants are encouraged to visit the Cancer Moonshot Scholars webpage to learn more.
Publications
Samimi G, House M, Benante K, Bengtson L, Budd T, Dermody B, DeShong K, Dyer V, Kimler BF, Sahasrabuddhe VV, Siminski S, Ford LG, Vilar E, Szabo E. Lessons Learned from the Impact of COVID-19 on NCI-sponsored Cancer Prevention Clinical Trials: Moving Toward Participant-centric Study Designs. Cancer Prev Res (Phila). 2022 May 3;15(5):279-284. doi: 10.1158/1940-6207.CAPR-21-0578. PMID: 35502553.
Active and DCP-Approved Studies
A list of active and DCP-approved studies is available on the Trials page on the CP-CTNet DMACC website. Each trial includes the DCP ID, clinicaltrials.gov ID, status (recruiting or not yet recruiting), the study start date, and a link to a trial-specific information page once trial information is available on clinicaltrials.gov.
Upcoming Events
Meetings and events can be found on the Meetings and Events page on the CP-CTNet DMACC website.
| Cycle # | Steering Committee Date | Concept Solicitation Date | Concept Due Date |
|---|---|---|---|
| 12 | July 29, 2022 | Aug. 5, 2022 | Oct. 5, 2022 |
| 13 | Oct. 28, 2022 | Nov. 4, 2022 | Jan. 9, 2023 |